
Agronomy, Without the Myths
Chloride in fertilisers: context matters more than content
Chloride problems in agriculture are often blamed on fertilisers. This article explains why organic fertilisers are rarely the real issue — and why water, mineral inputs and total chloride load matter far more


Chloride in organic fertilisers
Why it is rarely the real problem
Let’s start with something familiar.
Plants are a bit like people:
they need a small amount of salt to function properly.
Too little, and basic processes slow down.
Too much, and problems appear quickly.
So yes — chloride matters.
But the real issue is not its presence, it’s its accumulation.
That distinction is often lost.
This article explains why.
First things first: what is chloride?
Chloride (Cl⁻) is:
a negatively charged ion
fully water-soluble
hardly bound to soil particles
present mainly in soil water, not in the solid soil phase
From an agronomic perspective, chloride:
does not build soil structure
is rarely applied intentionally as a nutrient
behaves largely as a passenger, moving wherever water moves
Or more simply:
Plants don’t “see” fertilisers.
They respond to ion concentrations in the root zone.
This is fundamental to understanding chloride behaviour.
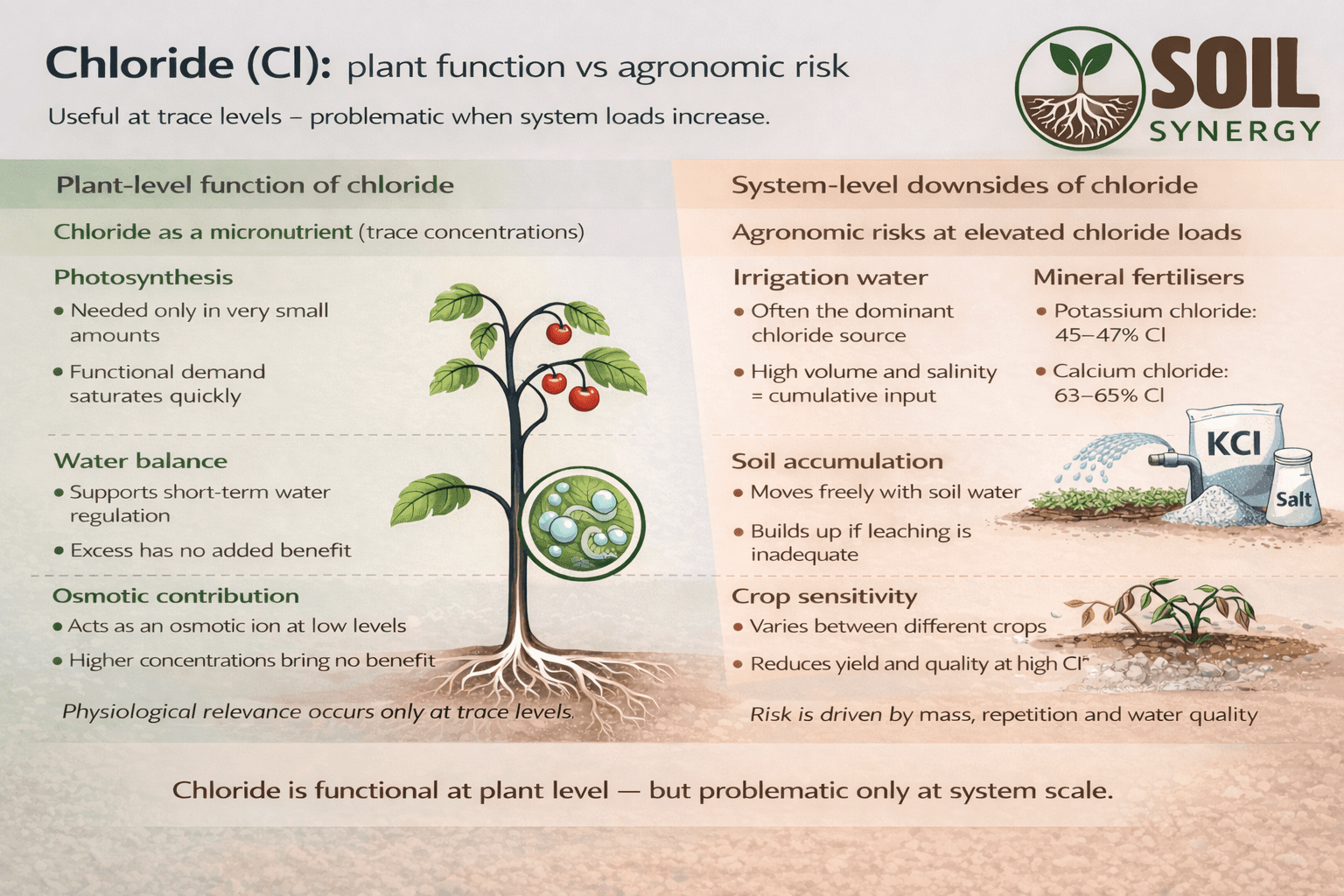
Chloride at plant level: useful, but only in traces
Chloride is officially classified as an essential micronutrient.
At very low concentrations, plants actively use chloride as part of their physiological machinery. Research shows that chloride contributes to:
normal photosynthetic functioning
osmotic regulation at cellular level
maintaining ionic balance alongside potassium and calcium
Chloride deficiency can occur, but under practical field conditions it is rare.
Where it does appear, reported symptoms include:
wilting despite sufficient water
chlorosis and reduced leaf expansion
excessive lateral root branching
These symptoms are often mistaken for nitrogen or potassium deficiencies, which makes correct diagnosis important.
The key point:
The physiological demand for chloride is extremely small and saturates quickly.
Beyond trace levels, additional chloride does not improve plant function.
When chloride becomes a problem
Most chloride-related issues are not biological, but systemic.
At elevated system loads, chloride can:
accumulate in the root zone
interfere with nitrate uptake
reduce yield and quality
cause leaf tip burn and marginal necrosis
Chloride toxicity is far more common than deficiency, especially in systems with saline irrigation water or repeated chloride inputs.
Crop sensitivity also matters:
Chloride-sensitive crops include beans, berries, grapes, potatoes and many greenhouse crops
More tolerant crops include barley, sugar beet and some forage grasses
Chloride management is therefore crop-specific, not ideological.
The real question you should ask
Not:
“Does my fertiliser contain chloride?”
But:
“How much chloride enters my system in total?”
This is where many discussions go wrong.
How much chloride comes from where?
Let’s look at order of magnitude, not labels.
Indicative chloride inputs in agriculture
Source | Chloride (Cl) | Comment |
|---|---|---|
Organic fertiliser (poultry manure pellets) | 0.2–0.7 % | Highly variable |
Next-generation organic fertiliser | ~0.75 % | Slightly higher, same order |
Potassium chloride (KCl / MOP) | ~45–47 % | Very high chloride input |
Calcium chloride (CaCl₂) | ~63–65 % | Extremely concentrated |
Rainwater | <10 mg/L | Practically chloride-free |
Fresh irrigation water | 20–150 mg/L | Often underestimated |
Brackish irrigation water | 200–1,000+ mg/L | Where problems start |
Putting this into perspective
The difference between:
0.5 % chloride
and 0.75 % chloride
in an organic fertiliser is negligible compared to:
repeated use of KCl-based fertilisers
years of chloride-containing NPK applications
large volumes of irrigation water
Or put differently:
If your system already receives hundreds of kilograms of chloride per hectare per year from water or mineral fertilisers,
a few extra kilograms from organic fertiliser will not make or break the system.
Water: the silent chloride contributor
This factor is frequently underestimated.
A simple calculation:
5,000 m³ irrigation water per hectare
× 200 mg chloride per litre (moderately brackish)
= 1,000 kg chloride per hectare per year
That is:
far more than organic fertilisers contribute
often more than fertilisers combined
Chloride is mobile, which means it can be leached — but only under the right conditions.
Leaching works when:
drainage is sufficient
irrigation water itself is low in chloride
Leaching saline soils with saline water
is not leaching — it’s redistribution.

“But organic fertilisers help desalination, right?”
Indirectly — yes.
But with clear limits.
Organic matter can:
improve soil structure
increase infiltration
enhance water movement through the soil profile
This improves the system’s capacity to flush salts out — if water quality allows it.
But:
Organic fertilisers do not remove chloride.
They only enable removal under the right conditions.
No clean water = no real desalination.
Practical chloride management: what actually helps
Effective chloride management focuses on system controls, not individual products:
Water quality first
Irrigation water largely determines the chloride ceiling of a system.Mineral fertiliser source selection
Sulphate- or nitrate-based potassium sources matter far more than minor chloride differences in organic inputs.Soil monitoring
Chloride must be interpreted in relation to texture, drainage and irrigation history.Organic matter as an enabler, not a solution
Organic inputs improve structure and infiltration but do not neutralise chloride.
Why chloride in organic fertilisers is rarely decisive
Even if:
a next-generation organic fertiliser
contains slightly more chloride
than conventional poultry manure pellets
the reality remains:
absolute chloride input is small
application rates are limited
system impact is marginal
Chloride issues are driven by:
volume
repetition
water quality
Not by one organic product.
Conclusion
Chloride is everywhere.
The real question is not whether chloride is present,
but whether it accumulates to levels that harm soil and crops.
Focusing on the chloride content of a single fertiliser
while ignoring:
irrigation water
fertiliser history
total chloride load
means missing the real drivers.
Organic fertilisers can fit perfectly well into a sound system.
But the idea that “a bit of chloride in organic fertiliser” is decisive
simply doesn’t hold up.
Chloride itself is not the enemy.
Poor system design is.
Sources
FAO – Water Quality for Agriculture (Ayers & Westcot)
FAO – *Salt-affected soils and their management
Havlin et al. - Soil Fertility and Fertilizers: An Introduction to Nutrient Management
Ayers, R.S. & Westcot, D.W. (FAO) - Water Quality for Agriculture -FAO Irrigation and Drainage Paper 29
Chloride in organic fertilisers
Why it is rarely the real problem
Let’s start with something familiar.
Plants are a bit like people:
they need a small amount of salt to function properly.
Too little, and basic processes slow down.
Too much, and problems appear quickly.
So yes — chloride matters.
But the real issue is not its presence, it’s its accumulation.
That distinction is often lost.
This article explains why.
First things first: what is chloride?
Chloride (Cl⁻) is:
a negatively charged ion
fully water-soluble
hardly bound to soil particles
present mainly in soil water, not in the solid soil phase
From an agronomic perspective, chloride:
does not build soil structure
is rarely applied intentionally as a nutrient
behaves largely as a passenger, moving wherever water moves
Or more simply:
Plants don’t “see” fertilisers.
They respond to ion concentrations in the root zone.
This is fundamental to understanding chloride behaviour.

Chloride at plant level: useful, but only in traces
Chloride is officially classified as an essential micronutrient.
At very low concentrations, plants actively use chloride as part of their physiological machinery. Research shows that chloride contributes to:
normal photosynthetic functioning
osmotic regulation at cellular level
maintaining ionic balance alongside potassium and calcium
Chloride deficiency can occur, but under practical field conditions it is rare.
Where it does appear, reported symptoms include:
wilting despite sufficient water
chlorosis and reduced leaf expansion
excessive lateral root branching
These symptoms are often mistaken for nitrogen or potassium deficiencies, which makes correct diagnosis important.
The key point:
The physiological demand for chloride is extremely small and saturates quickly.
Beyond trace levels, additional chloride does not improve plant function.
When chloride becomes a problem
Most chloride-related issues are not biological, but systemic.
At elevated system loads, chloride can:
accumulate in the root zone
interfere with nitrate uptake
reduce yield and quality
cause leaf tip burn and marginal necrosis
Chloride toxicity is far more common than deficiency, especially in systems with saline irrigation water or repeated chloride inputs.
Crop sensitivity also matters:
Chloride-sensitive crops include beans, berries, grapes, potatoes and many greenhouse crops
More tolerant crops include barley, sugar beet and some forage grasses
Chloride management is therefore crop-specific, not ideological.
The real question you should ask
Not:
“Does my fertiliser contain chloride?”
But:
“How much chloride enters my system in total?”
This is where many discussions go wrong.
How much chloride comes from where?
Let’s look at order of magnitude, not labels.
Indicative chloride inputs in agriculture
Source | Chloride (Cl) | Comment |
|---|---|---|
Organic fertiliser (poultry manure pellets) | 0.2–0.7 % | Highly variable |
Next-generation organic fertiliser | ~0.75 % | Slightly higher, same order |
Potassium chloride (KCl / MOP) | ~45–47 % | Very high chloride input |
Calcium chloride (CaCl₂) | ~63–65 % | Extremely concentrated |
Rainwater | <10 mg/L | Practically chloride-free |
Fresh irrigation water | 20–150 mg/L | Often underestimated |
Brackish irrigation water | 200–1,000+ mg/L | Where problems start |
Putting this into perspective
The difference between:
0.5 % chloride
and 0.75 % chloride
in an organic fertiliser is negligible compared to:
repeated use of KCl-based fertilisers
years of chloride-containing NPK applications
large volumes of irrigation water
Or put differently:
If your system already receives hundreds of kilograms of chloride per hectare per year from water or mineral fertilisers,
a few extra kilograms from organic fertiliser will not make or break the system.
Water: the silent chloride contributor
This factor is frequently underestimated.
A simple calculation:
5,000 m³ irrigation water per hectare
× 200 mg chloride per litre (moderately brackish)
= 1,000 kg chloride per hectare per year
That is:
far more than organic fertilisers contribute
often more than fertilisers combined
Chloride is mobile, which means it can be leached — but only under the right conditions.
Leaching works when:
drainage is sufficient
irrigation water itself is low in chloride
Leaching saline soils with saline water
is not leaching — it’s redistribution.

“But organic fertilisers help desalination, right?”
Indirectly — yes.
But with clear limits.
Organic matter can:
improve soil structure
increase infiltration
enhance water movement through the soil profile
This improves the system’s capacity to flush salts out — if water quality allows it.
But:
Organic fertilisers do not remove chloride.
They only enable removal under the right conditions.
No clean water = no real desalination.
Practical chloride management: what actually helps
Effective chloride management focuses on system controls, not individual products:
Water quality first
Irrigation water largely determines the chloride ceiling of a system.Mineral fertiliser source selection
Sulphate- or nitrate-based potassium sources matter far more than minor chloride differences in organic inputs.Soil monitoring
Chloride must be interpreted in relation to texture, drainage and irrigation history.Organic matter as an enabler, not a solution
Organic inputs improve structure and infiltration but do not neutralise chloride.
Why chloride in organic fertilisers is rarely decisive
Even if:
a next-generation organic fertiliser
contains slightly more chloride
than conventional poultry manure pellets
the reality remains:
absolute chloride input is small
application rates are limited
system impact is marginal
Chloride issues are driven by:
volume
repetition
water quality
Not by one organic product.
Conclusion
Chloride is everywhere.
The real question is not whether chloride is present,
but whether it accumulates to levels that harm soil and crops.
Focusing on the chloride content of a single fertiliser
while ignoring:
irrigation water
fertiliser history
total chloride load
means missing the real drivers.
Organic fertilisers can fit perfectly well into a sound system.
But the idea that “a bit of chloride in organic fertiliser” is decisive
simply doesn’t hold up.
Chloride itself is not the enemy.
Poor system design is.
Sources
FAO – Water Quality for Agriculture (Ayers & Westcot)
FAO – *Salt-affected soils and their management
Havlin et al. - Soil Fertility and Fertilizers: An Introduction to Nutrient Management
Ayers, R.S. & Westcot, D.W. (FAO) - Water Quality for Agriculture -FAO Irrigation and Drainage Paper 29
Related posts



